Reformate is a blending component that makes up about 30 percent of US gasoline supplies. It is also an important source of aromatics used as feedstocks for the petrochemicals industry. Ongoing changes in the US crude oil slate are reducing the volume of heavy naphtha available to feed catalytic reformer units that make reformate. At the same time better economics for lighter ethane feedstock are reducing the volume of aromatics produced as byproducts of olefin cracking. The result is a shortage of the aromatic materials used to produce a number of petrochemical intermediates such as polymers and fibers. But more changes are coming to the reformate market due to reductions in the use of reformate in gasoline. Today we look at the changing role of reformate.
|
Check out Kyle Cooper’s weekly view of natural gas markets at |
Reformate is primarily used as a gasoline blending component but it is also used as a petrochemical feedstock. Reformate produced from refinery naphtha accounts for about 30 percent of the US gasoline pool (source: ExxonMobil). As we have discussed in previous blogs, gasoline is a complicated cocktail that refiners blend to meet a myriad of quality and regulatory specifications depending on geographic and weather conditions using a number of components often referred to as the gasoline blending pool. Several previous RBN blogs touch on reformate and gasoline blending. We covered refinery processes in our “Complex Refining 101 Part I and Part II” series. We have covered alkylate - another important component of the gasoline blending pool (see The Octane Boost in Gasoline Blending). We covered the Reid Vapor Pressure (RVP) quality characteristic of gasoline in “Regulatory Gas Pressure Party”. RVP levels are mandated by the Environmental Protection Agency (EPA) and apply during the summer driving season. One reformate quality is that it helps refiners meet lower RVP mandates during the summer months. [We covered the opposite quality of normal butane blended into gasoline during the winter months in “Wasted Away in Butaneville”] We have also covered the Renewable Fuels Standard (RFS) that requires refiners to blend ethanol into gasoline (see “Crushing Pain and Mandate Madness” and “Ethanol Mandates, Motor Gasoline and the Blend Wall”) as well as new regulations proposing Tier 3 sulfur standards for gasoline expected to go into effect in 2017 (see The Tears of a Refiner). More recently we concluded a series on the US condensate market with a blog about demand for naphtha produced by condensate splitters (see The Market for US Gulf Naphtha).
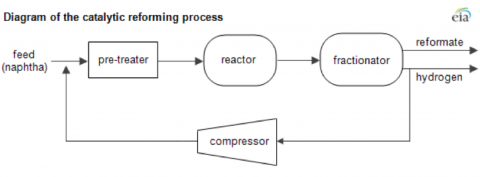
Reformate is produced in refinery catalytic reformer units using heavy range naphtha feedstock produced by “straight run” crude distillation (see Figure 1). The naphtha is pre-treated to remove sulfur and nitrates. The reformer unit uses catalysts to reform (meaning rearrange the molecules of) heavy naphtha from a low octane refined product into reformate – a high-octane gasoline component. Octane improves gas engine performance by reducing engine “knock” (see The Octane Boost in Gasoline Blending). Besides reformate, catalytic reforming of heavy naphtha produces a number of byproducts – the most significant being hydrogen gas (used in a number of other refinery processes -see for example Pump up the Volume ). Smaller quantities of natural gas liquids are also produced including isobutane that can be used as a feedstock for refinery alkylation.
Figure 1
Source: EIA (Click to Enlarge)
Reformate has an API gravity range of between 30 and 55, a very low RVP level of 0.5-2.5 psi, and a relatively low sulfur content of a maximum 0.5 percent. These qualities make it an ideal blending component for gasoline but reformate also contains the aromatic product components benzene, toluene and xylene - known collectively as BTX that are used in the manufacture of certain petrochemical intermediates. To this end aromatic components can be removed from reformate using a solvent extraction process. Figure 2 is a schematic flow diagram for the extraction of BTX aromatics from reformate. Aromatics like BTX are used in the production of specific types of plastics such as polymers and fibers. We’ll get back to petrochemicals in a minute but first we turn to changes in the US gasoline and crude markets that are impacting the extent to which reformate continues to be used as a gasoline blending component.
Join Backstage Pass to Read Full Article