Alkylate is a valuable blending component that accounts for about 12 percent of the US gasoline pool. Alkylate is manufactured by combining elements derived from NGLs and crude oil refining and is an important link between these two hydrocarbon markets. Alkylate has critical qualities required to meet complex modern gasoline quality specifications. Today we look at the qualities and manufacture of alkylate.
US Gasoline is a complicated product that refiners blend to meet local quality and regulatory specifications using a number of components often referred to as the gasoline blending pool. Alkylate is an important gasoline blending component that has little name recognition outside refinery circles. A number of previous RBN blogs touch on the alkylate story. We covered refinery processes in our “Complex Refining 101 Part I and Part II” series. We covered the Reid Vapor Pressure (RVP) quality characteristic of gasoline in “Regulatory Gas Pressure Party”. RVP levels are mandated by the Environmental Protection Agency (EPA) and apply during the summer driving season. One alkylate quality is that it helps refiners meet lower RVP mandates during the summer months. [We covered the opposite quality of butane blended into gasoline during the winter months in “Wasted Away in Butaneville”] We have also covered the Renewable Fuels Standard (RFS) that requires refiners to blend ethanol into gasoline (see “The RIN and Stimpy Show – Crushing Pain and Mandate Madness” and “A Market of Contradictions: Ethanol Mandates, Motor Gasoline and the Blend Wall”).
Alkylate is particularly prized for its high octane, low RVP and low sulfur properties. In the US alkylate is responsible for about 11-13 percent of the gasoline pool depending on regional demand and seasonal factors (Worldwide Refinery Processing Review, 2011). The January 2012 EIA refinery capacity report indicates that US alkylate production capacity is 1.3 MMb/d compared to total US refinery capacity of 17.3 MMb/d. Alkylate is more expensive than gasoline. Over the past two years US Gulf alkylate prices have averaged 30 cnts/Gal over unleaded gasoline.
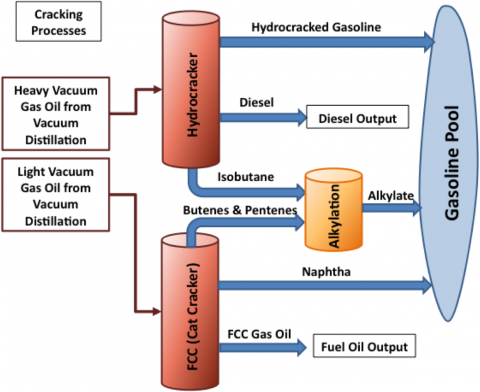
Alkylate is manufactured in refinery alkylation units, informally shortened to “alky” units. Alkylation is a chemical process where the light gaseous hydrocarbons butylene or propylene and isobutane are combined together to make heavier high-octane alkylate. The three light hydrocarbons used as feedstocks to the alkylation process are primarily produced by refinery cracking processes (see diagram below). The refinery cracking process breaks down light and heavy gasoil derived from vacuum distillation (see Complex Refining 101 Part 2). Butylene and propylene are olefins (a particular class of oil molecule having two hydrogen atoms for each carbon atom) output from refinery fluid catalytic cracking (FCC) units. Isobutane is an output from refinery hydrocrackers that break down heavier vacuum gasoil. Significant volumes of isobutane are produced at gas processing plants and split out of “y-grade” or mixed NGL streams in NGL fractionation plants. Isobutane is also produced by converting normal butane in a butamer (a.k.a., isomerization) unit. The butylene or propylene and isobutane are fed into a reactor, where - under the influence of a sulfuric-acid or hydrofluoric-acid catalyst -they combine
to form a mixture of heavier hydrocarbons. The liquid fractionate of this mixture of compounds is called alkylate.
Source: RBN Energy (Click to Enlarge)
To understand the part alkylate plays in the gasoline that ends up in your tank at the gas station, we need to run through a few gasoline characteristics:
- Gasoline is manufactured by refineries and product blenders using different recipes that vary - sometimes significantly because of:
- Regulation: or mandates that control certain gasoline blending components or product qualities such as oxygenates (Clean Air Act), ethanol (Renewable Fuel Standards), seasonal RVP limits, octane ratings (Motor Vehicle Manufacturers) and even the entire recipe for gasoline (EPA reformulated gasoline, RFG).
- Location: where the gasoline is sold determines the quality specification and that can be different depending on regions, States (for example California) or by country.
- Gasoline is a composite product – a blend of hydrocarbons designed to meet particular market requirements
- Most gasoline components are the result of refining processes but some like ethanol are manufactured entirely separately. Butane, isobutane and natural gasoline come from natural gas fractionation as well as petroleum refining. Other complex gasoline components are produced as byproducts from petrochemical olefin crackers.
- Every refinery is configured differently and can use different processes to extract gasoline components from crude oil or other feed stocks
- Different crude oils produce different quantities of gasoline components in different refinery configurations
Alkylate produced by alkylation is therefore just one of a number of components that refiners blend into their gasoline cocktail in order to develop the properties required to meet prevailing quality and blend regulations in a particular market. The most important alkylate blending qualities in this respect are its high octane rating (94) and low RVP (4.0 pounds per square inch). Alkylate also has a very low sulfur content. These qualities make alkylate particularly attractive for blending today’s clean burning quality gasoline.
We have covered RVP properties and regulations previously (see “Regulatory Gas Pressure Party “ and “Wasted Away in Butaneville”. Octane rating is a measure of gasoline performance. Most gasoline fuel autos have four-stroke engines. One of the strokes is the compression stroke, where the engine compresses a cylinder-full of air and gas into a much smaller volume before igniting it with a spark plug. The amount of compression is called the compression ratio of the engine. A typical auto engine might have a compression ratio of 8-to-1. The octane rating of gasoline tells you how much the fuel can be compressed before it spontaneously ignites. When gas ignites by compression rather than because of the spark from the spark plug, it causes knocking in the engine. Knocking can damage an engine (and make the ride uncomfortable). Lower-octane gas (like "regular" 87-octane gasoline) can handle the least amount of compression before igniting. Octane ratings are industry standard measures of the anti knocking capabilities of gasoline. In the US two indexes are used – the Research Octane Number (RON) and the Motor Octane Number (MON). You may have seen “(R+M)/2” displayed on the gas pump while you were watching it suck up your dollars. The US standard for gasoline is 87 octane for regular and 92/93 octane for premium. Premium gasoline costs a bunch more and is “required” in fancy autos because their more powerful engines run more smoothly with higher octane gas.
Refiners can use alkylate to boost octane rating when other gasoline components are low in octane. When refiners originally started to tweak the octane rating of gasoline, they did it by adding tetra ethyl lead – aka lead. That turned out to have nasty side effects on the intelligence of the population. Nowadays alkylate competes with ethanol for refinery blenders looking for higher octane.
Refiners balance their use of ethanol and alkylate in gasoline because each has different octane and RVP qualities as follows:
- Ethanol has a very high (R+M)/2 octane rating of 113 and if it is less expensive than gasoline it is preferable to alkylate as an octane enhancer
- Refiners typically only use up to 10 percent ethanol in US gasoline blends in part because auto engines are not designed to use much higher ethanol percentages
- Ethanol is not manufactured at refineries and is not transported to terminals by pipeline because it attracts water, so increasing the ethanol blend percentage creates logistic constraints.pipelines.
- Ethanol increases the RVP level in gasoline
- Alkylate has a very low RVP (4.0 PSI) so during the summer months when RVP limits apply refiners use alkylate to reduce high RVP levels in ethanol blends.
- Alkylate is more readily available to refiners and has good drivability properties in addition to high octane
- In the colder winter months, refiners look to blend more high RVP normal butane into gasoline because it is less expensive then gasoline. They increase alkylate in the gasoline blend to keep the overall RVP level down.
Gasoline contains a complex blend of components that provide different qualities at different times of the year and in different locations. Alkylate is just one component - albeit a valuable and flexible one with low RVP and high octane qualities. The process of alkylation blends together byproducts from NGLs (normal butane, isobutane) with olefins from the refinery FCC unit. This mixture of hydrocarbon products is an integral part of the link between NGL and crude oil markets
|
Each business day RBN Energy releases the Daily Energy Post covering some aspect of energy market dynamics. Receive the morning RBN Energy email by signing up for the RBN Energy Network. |
“We skipped the light fandango” is the first line of one of the most famous “one hit wonders” – the song “A Whiter Shade of Pale” released by Procol Harum in 1967









Comments
Butylene vs Propylene for U.S. Alkylate Production
For year 2013 (or any current year) what was the demand split between butylene and propylene consumed in alkylate production? I am unable to quantify, but it appears very little propylene is used in alkylate production. Only stranded propylene.